The Internet's Best Source For Water Well Drilling Information
| Driller Search | List Your Business |
| Worthwhile Causes |
| Well Drilling Pictures |
| Ask The Well Guys |
| David Thein |
| About Us |
Removal of hydrogen sulfide gas and iron in well water by the pressurized oxidation method
Joseph A. Lane
Technical Marketing Executive
Amtrol Inc. 9/21/04
Both hydrogen sulfide gas and iron in well water either together or alone create problems for the homeowner. Obnoxious odors and tastes of hydrogen sulfide gas and rust staining of fixtures due to iron are the result of these problems. Many solutions have been proposed but all with drawbacks. Most require the addition of chemicals that are expensive or are too slow to react to meet household demands. To overcome these problems, products have been developed that utilize mother- nature and lessons learned from the agriculture industry. At one time in was the common practice to eliminate crop, destroying insects by the use of toxic chemicals. It was later found out that mother nature can take care of the problem without the use of chemicals. Taking a cue from the agriculture industry, a new process has been developed to apply mother natureís technique to the water well industry to treat water with hydrogen sulfide gas and iron by supersaturating the water with air under high pressure. This method is referred to as pressurized oxidation. This differs from the present air induction systems by providing air under high pressures.
Hydrogen Sulfide Gas Removal
In nature, hydrogen sulfide (H2S) gas and oxygen (O2) canít co exist without a reaction. The natural chemical reaction is H2S + O2 = water (H2O) + elemental sulfur. Figure 1 depicts the chemical reaction.

Hydrogen Sulfide Gas + Oxygen = Water + Elemental Sulfur
Figure 1
Since the hydrogen sulfide gas exists in the water it is necessary to dissolve the air into the water in order for the natural reaction to take place. It has been determined by calculation and verified by tests that air can be dissolved up to 10X under pressure as great as air being dissolved at atmospheric pressure. This is the main reason that the reaction time with pressurized air is quicker. The various quantities of dissolved air at various pressures and temperatures are shown in Figure 2.
Solubility of Air in Water
Ratio of Absorbed Air Volume to Water Volume(expressed as a decimal)
Temp Pressure
| 0 F | 0 | 20 | 40 | 60 | 80 | 100 |
| 40 | .0258 | .0613 | .0967 | .1321 | .1676 | .2030 |
| 50 | .0223 | .0529 | .0836 | .1143 | .1449 | .1756 |
| 60 | .0197 | .0469 | .0742 | .1014 | .1296 | .1559 |
| 70 | .0177 | .0423 | .0669 | .0916 | .1162 | .1408 |
| 80 | .0161 | .0387 | .0614 | .0840 | .1067 | .1293 |
Figure 2
In the pressurized oxidation system, air is introduced into the water by means of an inductor. The system pressure is boosted for optimum air absorption. The water acts like a sponge and absorbs the high, pressure air. The high, pressure water that now contains the absorbed air and hydrogen sulfide gas begin to react. Since the reaction time is not immediate and is directly dependant on the ppm of the hydrogen sulfide (See Fig 3), contact volume is required. The total contact volume is dependant upon the demand and hydrogen sulfide gas in ppm of the system.
| Concentration of hydrogen Minutes of contact | Sulfide H2S in ppm required |
| 0 | 0 |
| 1 | 130 |
| 2 | 180 |
| 3 | 220 |
| 4 | 250 |
| 5 | 270 |
| 6 | 300 |
| 7 | 320 |
Figure 3
The inductor is capable of producing more air than is required. In the first treatment tank, an air release volume control exhausts the excessive air and maintains a defined water level. Since the reaction takes place in the water, the gas that remains is either nitrogen or oxygen. This entrained gas becomes liberated from the water at the faucet in the form of tiny bubbles. All hydrogen sulfide gas has reacted while in solution; thus no obnoxious sulfur odors. The elemental sulfur falls to the bottom of the treatment tank and can be removed periodically via a drain.
An important feature of the pressurized oxidation system is that when the water leaves the treatment tank, it continues to contain residual levels of oxygen. If the system water demand for whatever reason was to be increased to a level greater than what was anticipated and hydrogen sulfide is detected at the faucet, by just turning off the demand for a few minutes the odor will dissipate. This is due to the fact that the water right up to the faucet has sufficient oxygen to react with the hydrogen sulfide gas. What is lacking is contact time; thus, the entire piping system downstream from the treatment tank, become contact volume. This is a major advantage over chemical treatment that when the system is overrun, the complete volume of the system downstream from the treatment source is not treatable. Homeowners using the untreated water become discouraged and dissatisfied.
Iron Removal
There are existing methods on the market that are used to remove both soluble ferrous and insoluble ferric iron from the water. Sand/multimedia filtration is used to remove insoluble iron but canít remove the soluble iron unless a potassium permanganate or other oxidant chemical is used as a regenerant. Ion exchange softeners, if the water requires softening along with iron removal, can be used. This type of iron removal requires chemicals and can only effectively remove ferrous iron in relatively low concentrations. Ferric iron will result in fouling.
Oxidation has been determined to be effective in eliminating both forms of iron. There are three (3) methods of oxidation: chlorination, ozonation and aeration. Of the three, aeration is the most economical and requires no chemicals. Atmospheric aeration due to the low adsorption rate requires large retention tanks and result in high capital costs. Thus, the most effective and economical method to eliminate both forms of iron is by the pressurized oxidation method. The same pressurized oxidation system used to remove hydrogen sulfide gas can be utilized to treat both forms of iron. When the iron concentration exceeds 1ppm, media filter tanks are to be added with a treated water backwash automatically programmed into the system. (Untreated water will result in less efficient removal of iron) Unlike hydrogen sulfide treatment, the pH of the water for effective iron removal is to be between 7 and 9. By supersaturating the water with pressurized air, some of the dissolved iron (ferrous) becomes insoluble (ferric). The media in the filter tanks acts as an insoluble catalyst to enhance the remaining dissolved oxygen and the iron to be insoluble iron and become trapped by the fine filtering characteristics of the media. Figure 4 depicts the process of iron removal using the combination of pressurized oxidation, and media filter tanks.
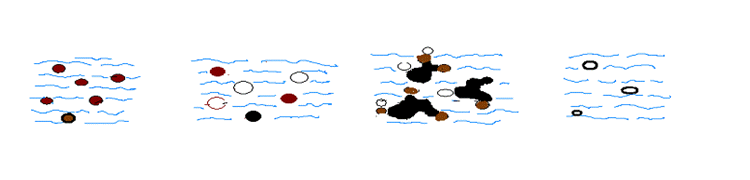
Pressurized oxidation
systems, when used with media filtration and backwashed daily with treated water, provides clear (ferrous) and precipitated ferric iron removal treatment up to 40 ppm and extends the life of the filter media. Figure 5 depicts a typical system. The prepiped, prewired, and preprogrammed controller, allows one valve at a time to sequentially operate and daily backwash treated water from the remaining two valves to drain. 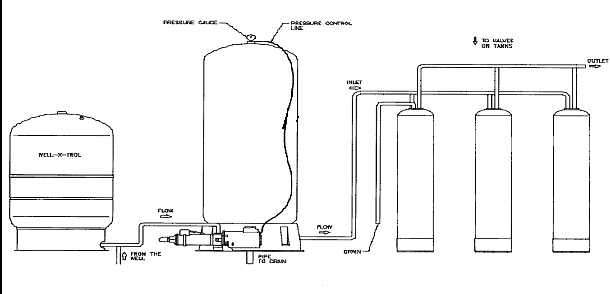
Figure 5
Although the pressurized oxidization system was initially developed for hydrogen sulfide removal, it has been easily converted to an iron removal system with the addition of the media filters. The addition of the preprogrammed valves for daily backwashing allows for high ppm of iron removal and the extension of the media life. Further modular features can be added to solve additional problems. You are limited only by your imagination.
Joseph A. Lane
Technical Marketing Executive
Amtrol Inc. 9/21/04
Joe Lane, Technical Marketing Executive for Amtrol Inc. has been in the water system industry for 40 years. He has a B.S. in Mechanical Engineering and hold 21 patents, including one for Amtrolís Well-X-trol
ģ line of pre-pressurized water well tanks. He is a former president of the Water System Council and is presently serving of the board of directors. He is also on the Board of Directors for the National Ground Water Association and recently received their Equipment Design Award. He is also a member of the Water Quality Association, the American Ground Water Trust, and numerous state water associations.
Copyright © 2006 Prime-Thein Enterprises All rights reserved.